Review of FOI F23-1799 and BC Public Health’s Handling of COVID-19 Vaccines AEFI – Part 1
COVID-19 Vaccines Harms: What and When BC Public Health Knew
Background
In April 2023, Lee Turner, of Doak Shireff Lawyers LLP, took over conduct of the defence of Dr. Charles Hoffe vs. the College of Physicians and Surgeons of BC. Mr. Turner subsequently filed a Freedom of Information (FOI) request (F23-1799) with the Provincial Health Services Authority (PHSA) to obtain data on Adverse Events Following Immunization (AEFI). FOI F23-1799 can be accessed at this link.
FOI F23-1799 was released in June 2024 and comprised over 1,300 pages of internal emails between BC CDC staff, Bonnie Henry, Reka Gustafson, and Monika Naus (then head of BC CDC), along with dozens of AEFI reports.
Upon reviewing FOI F23-1799, I noted key elements: discussions of AEFIs in emails, screenshots of non-public AEFI reports available through an intranet, and public-facing AEFI reports presented in chronological order. This arrangement made it possible to determine what BC public health officials like Bonnie Henry, the BC CDC and all 50+ medical health officers scattered over BC health authorities knew and when they knew it.
I reached an unsettling conclusion: BC CDC had manipulated the definition of Serious Adverse Events Following Immunization to lower the reported rates of Serious AEFIs in public-facing reports, thereby concealing the true risks associated with COVID-19 vaccines. I publicly shared this finding on June 14, 2024, in a comment on Byram Bridle’s post titled Breaking News: BC Centre for Disease Control Caught Lying and Withholding Important Public Health Data.
While my expertise is not in medical science, I specialize in detecting and documenting corporate and institutional misconduct. My background is in financial statement analysis. I worked as a hedge fund research analyst and compliance officer for a boutique investment firm. I have about 15 years of independent financial research and analysis experience. My focus is uncovering white-collar fraud. I’m not a forensic accountant by any measure; I’m just someone with somewhat odd proclivities for large sets of unstructured data and enjoy immersing myself in new topics.
The Case Against Dr. Hoffe
The College of Physicians and Surgeons of BC cancelled its February 11, 2022 citation against Dr. Hoffe on February 5, 2025. According to one media outlet, this was done because of a "material change of circumstances."
The College accused Dr. Hoffe of professional misconduct; specifically spreading misinformation about COVID-19 vaccines. Here’s an excerpt of the College’s accusations:
“… publicly expressing that the COVID-19 vaccinations cause microscopic blood clots that cause serious neurological harm, female infertility, and a high number of deaths that is not recognized by public health; …”
Many other doctors were publicly denouncing the COVID-19 vaccines and were persecuted by the College. However, the level of persecution against Dr. Charles Hoffe is particular. The College retained eight experts against Dr. Hoffe. Why is that?
In early 2021, Dr. Hoffe submitted a temporally- and vaccine lot-associated cluster of 11 AEFI reports to Interior Health, 10 of which involved Moderna lot #300042698, administered between January 18 and February 5, 2021.
As Dr. Hoffe began filing AEFI reports in early 2021—most notably in April and May— and went on public tours in BC warning the public about the harms of COVID-19 vaccines his actions posed a direct threat to a state narrative that sought to suppress information about vaccine-related harm. His findings challenged the political and ideological foundation underpinning the mass vaccination program and the totalitarian controls over the population that came along with it.
The evidence in FOI F23-1799 suggests that Bonnie Henry, the BC CDC, all health authorities, and all 50+ Medical Health Officers in BC were fully aware of these issues. Dr. Hoffe’s real "offence" was exposing what the BC government concealed from the public since early 2021. Dr. Hoffe’s AEFI reports constituted a cluster of AEFI associated with unexpected harms which required public health authorities to investigate and disclose to the public.
This post, and a few more to come, will cover my findings and analysis of how BC public authorities handled the COVID-19 vaccine AEFIs.
BC Public Health Officials Early Knowledge of Harms Associated with the COVID-19 Vaccines
Higher AEFI Rates Associated with Higher mRNA Content
January 28th, 2021 - P. 18, FOI F23-1799:
Above, is the upper half of an email to Bonnie Henry where Monika Naus, Medical Director of the BC CDC, states: “A history of anaphylaxis to a dose of the vaccine is a contraindication to receipt of future doses.” This is important to keep in mind as I will relate that statement to data in non-public AEFI reports further down.
Monika Naus, briefed Bonnie Henry and Martin Lavoie, Chief Medical Health Officer of Interior Health, on the observation that ‘cellulitis’ events occurred at “an appreciably higher rate” with the Moderna vaccine compared to Pfizer. However, she seems to downplay the significance of these reported cases.
Monika Naus explained that the higher rate of AEFIs associated with Moderna was consistent with its higher mRNA content. Her statements indicate an awareness that, since Pfizer and Moderna vaccines share similar structures, the key difference lies in mRNA dosage. Below is the lower half of Monika Naus’ email to Bonnie Henry.
Above, Monika Naus reports to Bonnie Henry the death of an inmate and two temporally associated thrombocytopenia reports. A few months later, the BC CDC will deny in public-facing AEFI reports that they received thrombocytopenia cases. Monika Naus stresses with capital letters that this is NOT being seen in the US analytic data comparing rates, indicating that she’s worried and that these findings in BC were unexpected. The link at the bottom of the above email is to a slide deck containing V-Safe data.
Misleading Public Reporting of Thrombocytopenia and Serious AEFIs
In the above email of January 28th, 2021 to Bonnie Henry, Monika Naus refers to two cases of temporally associated thrombocytopenia. Later, on p. 206 of F23-1799, the non-public AEFI report of March 25th, 2021, showed 3 cases of thrombocytopenia.
May 1st, 2021 – P. 287, FOI F23-1799:
Below, the public-facing BC CDC AEFI report from December 13th, 2020, to May 1st, 2021 stated:
“No safety signals have been identified in the reports received in BC to date”
“Serious events have not been reported at rates higher than expected compared to background rates.”
“There have been no reports of thrombosis with thrombocytopenia syndrome (TTS) reported in BC to date;”
The above-highlighted statements from the public-facing BC CDC AEFI report of May 1st, 2021, are misleading when compared to the non-public BC CDC AEFI reports.
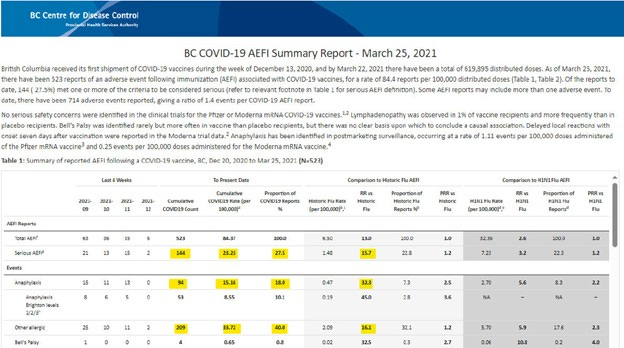
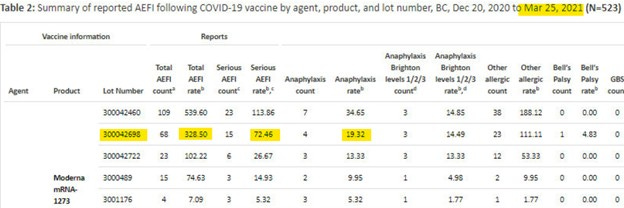
March 25th, 2021 – P. 205, FOI F23-1799:
Below,
The non-public BC CDC AEFI report shows 523 Total AEFI, 144 Serious AEFI, 94 Anaphylaxis AEFI and their respective rate per 100,000 doses are 84.37, 23.23, and 15.16.
Serious AEFI represent 27.5% of Total AEFI.
The COVID-19 vaccine Serious AEFI rate is unexpectedly 15.7 times greater than the historic flu vaccine Serious AEFI rate (background rate). This is an obvious safety signal.
The total AEFI rate of the historic flu is 6.5 per 100,000 doses
The Serious AEFI rate of the historic flu is 1.48 per 100,000 doses
Pandemic of the Newly Injected
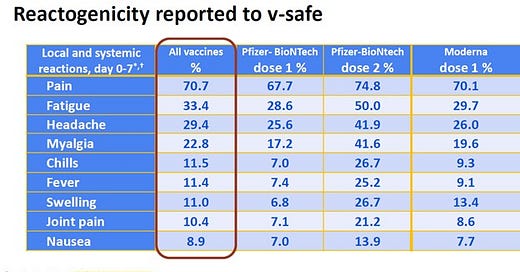
In the Monika Naus email of January 28th, 2021, there’s a link to a CDC slide deck that is no longer available for some reason. I managed to recover it. Its 7th slide shows AEFI from the V-Safe database that took New York Attorney Aaron Siri 18 months to obtain via the US courts. The slide shows US AEFI data as of January 14th, 2021, and the slide deck was published on January 27th, 2021.
Above is the sample size, which makes the numbers below extremely significant. The “All vaccines” red rectangle in the table below means All COVID-19 vaccines.
The above statistics were compiled from self-reporting recipients of the COVID-19 vaccines within 7 days after receipt of the vaccine. This is what you can expect within 7 days. I wonder if this is why Public Health Officials in BC and around the globe required you to wait between 14 days and 21 days before they would categorize you as being vaccinated?
*** The V-Safe data in the table above was in the possession of Bonnie Henry and the BC CDC since January 27th, 2021. ***
Comparing the AEFI rates between the first and second doses of Pfizer, it is evident that toxicity and harms increase with additional doses. We also see that when comparing dose 1 of Pfizer and Moderna, Moderna is more toxic than Pfizer. If the above level of risks had been disclosed to the public, it would have likely caused massive vaccine hesitancy in most sane people.
You can imagine that a large percentage of the population started having one or more of these very common reactions very early in the COVID-19 vaccine rollout and, believing the vaccines to be safe, attributed them to COVID-19 itself.
*** The COVID-19 vaccines caused a massive immediate surge in demand for healthcare. MASS INJURIES ***
The works of Denis Rancourt and Ed Dowd are what immediately come to my mind in support of mass injuries. Ed Dowd pointed out that the US Department of Labor maintained statistics on disabled people. Coinciding with the COVID-19 vaccine rollout we see a massive surge of people self-reporting as disabled. Here are the monthly year-over-year changes in disabilities observed by Ed Dowd.
The purpose of the non-sensical requirement that people needed to wait 14-21 days to be considered vaccinated was to shift the origin of the sudden massive demand for healthcare from the newly injected to the remaining unvaccinated and support propaganda designed to cause the public to demand and embrace restrictions on their personal freedom.
*** This massive sudden surge in demand for healthcare was propagandized as the pandemic of the unvaccinated. There never was a pandemic of the unvaccinated, it was a pandemic of the newly injected. ***
Proof of mRNA Toxicity and Harmfulness
Above, I highlighted the evidence that supports the conclusion that Monika Naus was aware in January 2021 that the AEFI rates increased with increased mMRA content. Below, we see data that was in the possession of BC public health officials demonstrating the mRNA toxicity and harmfulness.
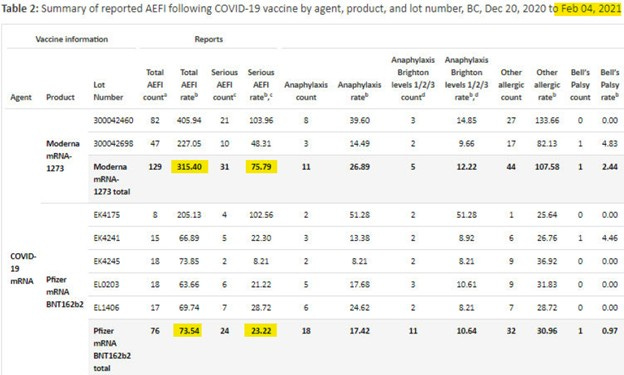
February 4th, 2021 – P. 42, F23-1799:
As explained by Monika Naus, the Pfizer and Moderna vaccines differ mainly by their mRNA content, 30 and 100 micrograms respectively. We can now think of Pfizer as a control for Moderna. Let’s crunch some ratios:
mRNA content of Moderna vs. Pfizer: 100 / 30 = 3.3
Total AEFI rate of Moderna vs. Pfizer: 315.40 / 73.54 = 4.3
Serious AEFI rate of Moderna vs. Pfizer: 75.79 / 23.22 = 3.3
This evidence suggests that mRNA is indeed a harmful substance. I first uncovered evidence to support this conclusion in 2021 when I calculated excess mortality from Canadian obituary data in the How I Became an Anti-Vaxxer in Q3 2021 post.
Monika Naus Warned Bonnie Henry Twice on March 11th, 2021
On March 11th, 2021, Monika Naus sent at least two emails to Bonnie Henry warning of her concern over serious AEFIs.
March 11th, 2021 – P. 151, F23-1799:
Monika Naus briefed Bonnie Henry that there was a safety signal for Bell’s Palsy, noting 4 cases vs. 2 expected relative to the flu vaccine. Monika announces that she is working on producing a report to be shared with the public. Monika’s email shows that MHOs (Medical Health Officers) also have access to the non-public BC CDC AEFI reports.
Monika Naus Warned Bonnie Henry of a Toxic Lot
March 11th, 2021 - P. 43, HTH-2021-14817:
Monika Naus briefed Bonnie Henry on a toxic lot that affected 12% of distributed vaccines in BC as of March 11th, 2021.
The 11 anaphylaxis AEFI per million dose rate (1.1 per 100,000) Monika Naus cites to Bonnie Henry is a benchmark of what can be expected. This email shows knowledge of a toxic Pfizer lot (EP6017) distributed throughout BC that has a calculated anaphylaxis AEFI rate of 40 per 100,000 doses. On March 11th, 2021, 294849 Pfizer doses had been distributed in BC.1 This affected 35,382 people in BC (12%).
Also, note that 15 of the 18 anaphylaxis cases were female which clearly shows a disproportionate risk to females of working age. The depth of the evidence demonstrating that BC public health officials were aware of the disproportionate COVID-19 vaccine harm to females will likely be covered in a future post.
Known Toxic Lots
Pfizer Lot EP6017
Recall that on January 18th, 2021, Monika Naus enunciated a standard of care and prudence by stating: “A history of anaphylaxis to a dose of the vaccine is a contraindication to receipt of future doses.”
On March 11th, 2021, Monika Naus warned Bonnie Henry that Pfizer lot EP6017 was showing an unexpectedly high rate of anaphylaxis. One of the rarest, but potentially deadly serious AEFI.
From all the non-public facing BC CDC AEFI reports in F23-1799, I compiled this table showing the number of cases of anaphylaxis reported for lot# EP6017:
EP6017 anaphylaxis cases formed a plateau from March 11th to April 8th, 2021, suggesting that the batch is exhausted, so you would think. But no, three more cases of anaphylaxis turned up between April 9th and September 15th, 2021. Given that anaphylaxis is a serious AEFI that occurs within 24 hours, this shows that despite their knowledge of the toxicity of this batch, the public health authorities failed to remove EP6017 from circulation.
On September 15th, 2021, EP6017 had a total count of AEFI of 71 and a rate of 126.48 AEFI per 100,000 doses. From this, we can infer that about 56,135 people received EP6017 in BC.
Other Toxic Lots
Pfizer lot# ER1742 is associated with 45 Serious AEFIs comprising 22 hospitalizations, 2 deaths, 23 anaphylaxis, and 22 anesthesia/paraesthesia. It was given to 222,258 people in BC as of September 15th, 2021.
Moderna lot# 3002179 is one of the 11 AEFI reports filed by Dr. Charles Hoffe. #3002179 had 14 serious AEFI reports comprising 6 associated hospitalizations. It was given to 84,063 people in BC as of September 15th, 2021.
Moderna lot# 300042698. 10 EAFI reports were filed against that lot by Dr. Charles Hoffe. #300042698 had 2 associated deaths, 10 Anaesthesia/paraesthesia, and one hospitalization. It was given to 20,903 people in BC as of September 15th, 2021.
The cluster of 10 unexpected AEFIs that Dr. Charles Hoffe reported to Interior Health is batch 300042698.
Batch 300042698 had:
A total AEFI rate of 328.5 per 100,000 doses
A serious AEFI rate of 72.46 per 100,000 doses
Batch 300042698 risk relative to the flu vaccine was 50.5x higher for any AEFI and 48.9x higher for Serious AEFI.
Moderna lot# 3001176 is associated with 15 serious AEFI reports comprising 6 hospitalizations. It was given to 59,973 people in BC as of September 15th, 2021.
Pfizer lot# EX0904 is associated with 24 Serious AEFIs comprising 7 hospitalizations, and 2 deaths. It was given to 119,227 people in BC as of September 15th, 2021.
Yet when Monika Naus prepared her public facing AEFI report concerning the Dec 13, 2020 to May 1, 2021 data, she made the following statements to the public:
“No safety signals have been identified in the reports received in BC to date”
“Serious events have not been reported at rates higher than expected compared to background rates.”
“There have been no reports of thrombosis with thrombocytopenia syndrome (TTS) reported in BC to date;”
In a subsequent post, I will show the extremely high variability of AEFI rates across dozens of vaccine lots monitored by the BC CDC which shows there was great variability in the toxicity of the various vaccine lots which should have caused public health officials to alert the public and to recall the toxic lots.
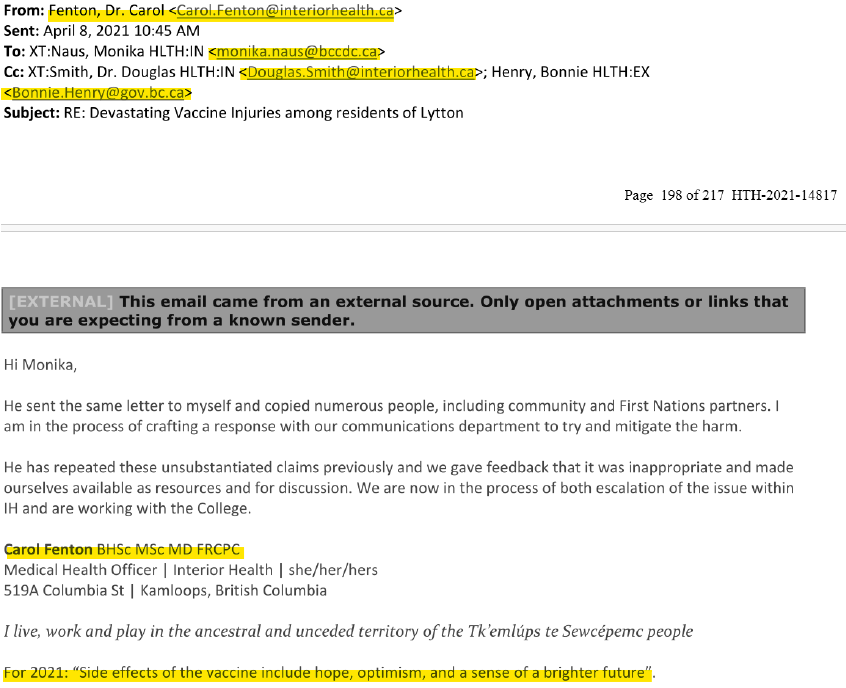
Public Health’s Reaction to Dr. Hoffe Expressing Concerns about injuries suffered by his patients after being injected with Moderna lot# 300042698
It is worth noting how Bonnie Henry and senior officials at Interior Health reacted to Dr. Hoffe expressing to Bonnie Henry about concerns he had as a result of the injuries he was witnessing in his patients following their receipt of the Covid-19 vaccine. Dr. Hoffe sent an email to Bonnie Henry on April 7, 2021 at 8:01 p.m. thanking her for the work she had been doing and expressing concern about the terrible side effects he had been seeing from the covid vaccines in his medical practice. On April 8, 2021 at 10:17 a.m. Monika Naus forwarded a copy of Dr. Hoffe’s email to Carol Fenton Medical Health Officer for Interior Health asking if she had any insight into his concerns and confirm that she was checking for AEFI reports among residents of Lytton, B.C. where Dr. Hoffe was practicing. On April 8, 2021 at 10:45 a.m. Carol Fenton said in an email to Bonnie Henry, and Douglas Smith, Executive Director for IH, that she was "in the process of crafting a response with our communications department to try and mitigate the harm." caused by Dr. Hoffe raising these concerns. She indicated that she was escalating the issue within Interior Health and was working with the College of Physicians and Surgeons. At 11:02 a.m. Bonnie Henry replied by email stating that she believed that Dr. Hoffe should be reported to the College. 25 minutes later, at 11:27 a.m., Douglas Smith confirmed that he had filed a formal complaint against Dr. Hoffe with the College “on behalf of the patients and communities affected by the actions of Dr. Hoffe.” It would appear that despite Bonnie Henry and Interior Health being aware of the toxicity of Moderna Lot# 300042698, and the increased level of harms being suffered across the province by those who had received a vaccine from that lot, and the fact that 10 of the 11 patients Dr. Hoffe had filed AEFI reports for had also received a vaccine from that lot, that his conduct in raising concerns was worthy of investigation and discipline for professional misconduct by the College.
Anaphylaxis Cases Management
Given Monika Naus stated: “A history of anaphylaxis to a dose of the vaccine is a contraindication to receipt of future doses.”, we should expect that a recommendation of no further immunization would be issued for each case of anaphylaxis. From all the non-public facing BC CDC AEFI reports I compiled this table shows the number of cases of anaphylaxis cases vs. the number of recommendations for no further immunizations.
On September 15th, 2021, Pfizer Lot EP6017 had tallied a total of 25 Serious AEFIs, 23 (92%) out of 25 were anaphylaxis cases. This is a public health hazard, and it should have been proactively disclosed to the public, or at the very least, every recipient who had received an injection from that lot should have received a letter from the Ministry of Health advising them that they have been exposed to a harmful substance and be followed by their family doctor accordingly.
We see that as of September 15th, 2021, just as the coercive vaccine mandates were being put in place, even if you had a case of anaphylaxis with the first dose, you had 68.6% chance of still being forced to take the second dose. The mere fact that the number of recommendations for no further immunization does not at least match the number of anaphylaxis cases, demonstrates that the COVID-19 vaccination rollout was never about public health.
Conclusion
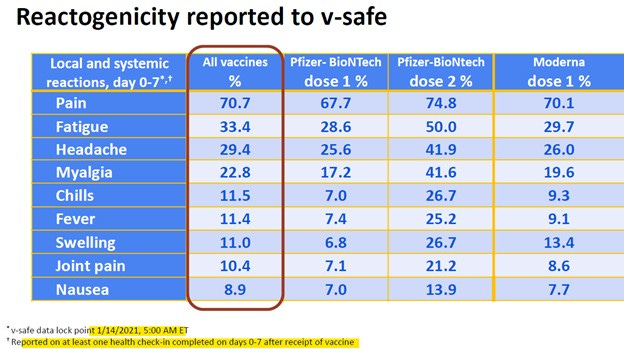
On January 27, 2021, the CDC’s COVID-19 Vaccine Safety Update (authored by Tom Shimabukuro, slides 7–9) reported high percentages of non-serious Adverse Events Following Immunization (AEFIs) from the V-Safe system, which Bonnie Henry and the BC CDC were aware of. These percentages can be converted to rates per 100,000 doses and compared to historical flu vaccine AEFI rates, which serve as a background benchmark.
The historical flu vaccine non-serious AEFI rate is calculated as 5.12 per 100,000 doses (total AEFI rate of 6.63 minus serious AEFI rate of 1.51). To assess the relative risk (RR) of non-serious AEFIs from COVID-19 vaccines compared to the flu vaccine, the V-Safe rates per 100,000 doses (derived from slides 7–9) are divided by 5.12. This calculation reveals how much riskier the COVID-19 vaccines were compared to the flu vaccine as of January 27, 2021.
The table below presents:
Left side: Common and very common non-serious AEFIs from V-Safe (slides 7–9) converted from percentages to rates per 100,000 doses.
Right side: Relative risk of these AEFIs, calculated by dividing the V-Safe rates per 100,000 doses by the flu vaccine’s non-serious AEFI rate of 5.12.
*** When AEFI rates are unexpectedly high relative to a background rate, that’s a safety signal. ***
By combining the BC CDC’s own data of Historic Flu AEFI rates with the rates of common and very common AEFIs from the V-Safe database, we see that within 7 days, with the COVID-19 vaccines, you were:
1381 times more likely to experience pain, and/or,
652 times more likely to experience fatigue, and/or,
574 times more likely to experience headaches, and/or,
445 times more likely to experience Myalgia, and/or,
225 times more likely to experience Chills, and/or,
223 times more likely to experience Fever, and/or,
215 times more likely to experience Swelling, and/or,
203 times more likely to experience Joint Pain, and/or,
174 times more likely to experience pain.
than you were with a flu shot.
Would anyone have agreed to be injected with a substance, if they were aware it had the above harm profile?
Safe and effective.
P. 139 FOI F23-1799: Total Pfizer AEFI reports = 249; AEFI rate per 100,000 doses = 84.45; Total # of Pfizer doses administered in BC on March 11th 2021 = 249 / 84.45 * 100,000 = 294849;